
The complaint also names Allergan plc, the parent company of Watson, and Endo International plc, the parent company of Endo Pharmaceuticals Inc. As a result, Watson made hundreds of millions of dollars more in generic Lidoderm sales. This agreement left Watson as the only generic version of Lidoderm on the market, substantially reducing competition and increasing prices for generic lidocaine patches. Endo and Watson illegally agreed that, for 7½ months after September 2013 (including the 180-day first-filer exclusivity period for which Watson was eligible), Endo would not compete by marketing an authorized generic version of Lidoderm.In 2012, Lidoderm sales in the United States approached $1 billion. As a result, Endo illegally maintained its monopoly over Lidoderm.
FTC SUIT AGAINST ENDO PHARMA FREE
In exchange, Endo paid Watson hundreds of millions of dollars, including $96 million of free branded Lidoderm product that Endo and Teikoku gave to Watson. that until September 2013, Watson would not compete with Endo and Teikoku by marketing a generic version of Endo’s Lidoderm patch. and Teikoku Pharma USA, Inc., illegally agreed with Watson Laboratories, Inc. In May 2012, Endo and its partners, Teikoku Seiyaku Co.In 2010, Opana ER sales in the United States exceeded $250 million. Endo used this period of delay to transition patients to a new formulation of Opana ER, thereby maintaining its monopoly power even after Impax’s generic entry. In exchange, Endo paid Impax more than $112 million, including $10 million under a development and co-promotion agreement signed during the same time period.
FTC SUIT AGAINST ENDO PHARMA PATCH
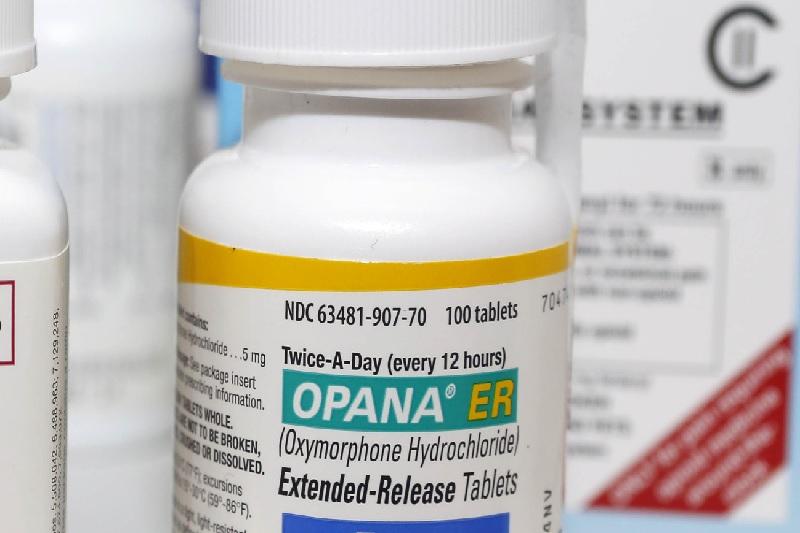
Lidoderm is a topical patch used to relieve pain associated with post-herpetic neuralgia, a complication of shingles. Opana ER is an extended-release opioid used to relieve moderate to severe pain. – to eliminate the risk of competition for Opana ER and Lidoderm, in violation of the Federal Trade Commission Act.
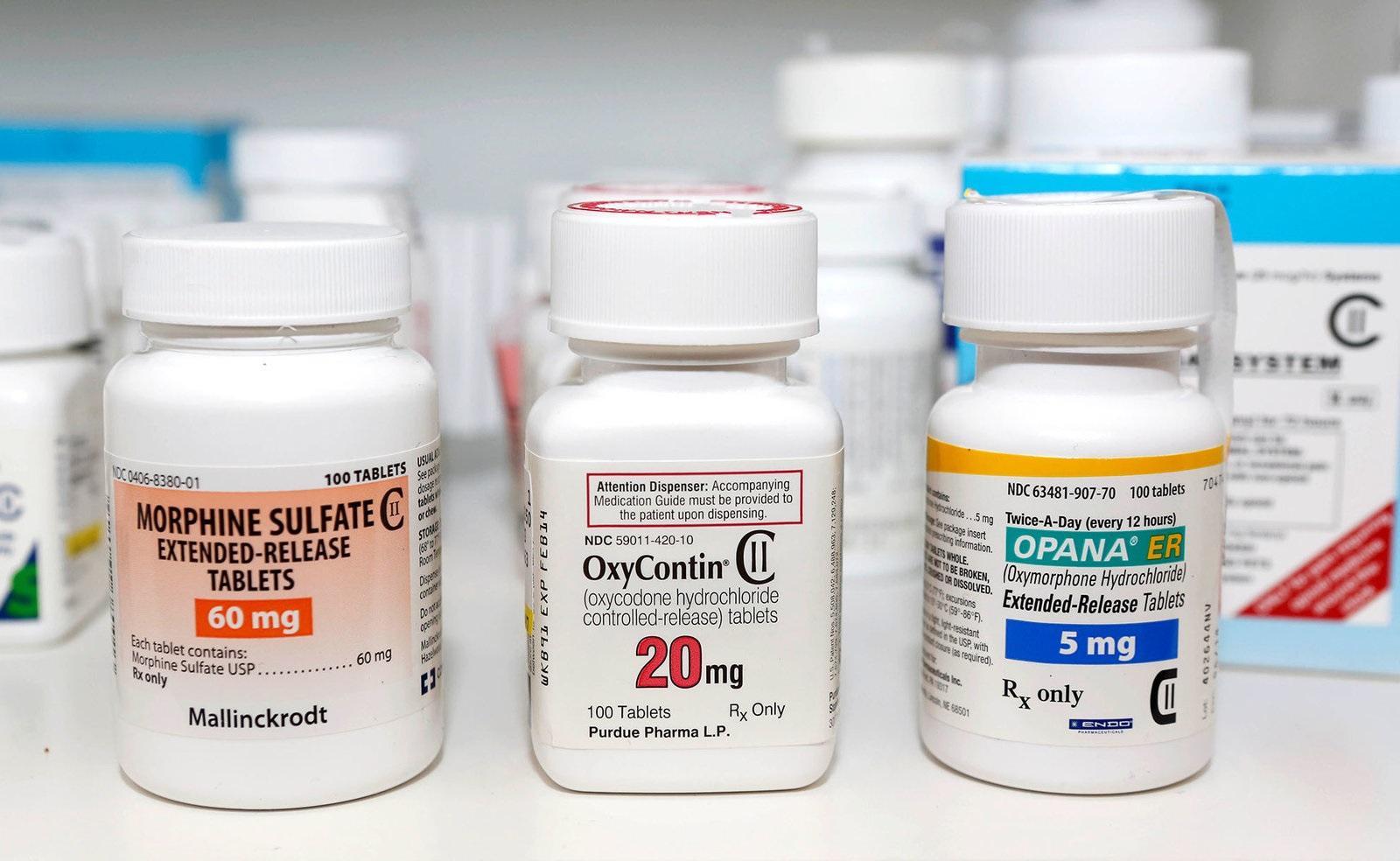
The FTC’s complaint alleges that Endo paid the first generic companies that filed for FDA approval – Impax Laboratories, Inc. “This lawsuit reflects the FTC’s commitment to stopping pay-for-delay agreements that inflate the prices of prescription drugs and harm competition, regardless of the form they take.” “Settlements between drug firms that include ‘no-AG commitments’ harm consumers twice – first by delaying the entry of generic drugs and then by preventing additional generic competition in the market following generic entry,” said FTC Chairwoman Edith Ramirez. and several other drug companies violated antitrust laws by using pay-for-delay settlements to block consumers’ access to lower-cost generic versions of Opana ER and Lidoderm.įollowing more than a decade of FTC challenges to pay-for-delay settlements, today’s enforcement action is the first FTC case challenging an agreement not to market an authorized generic – often called a “no-AG commitment” – as a form of reverse payment. The Federal Trade Commission filed a complaint in federal district court alleging that Endo Pharmaceuticals Inc.


Enforcement Show/hide Enforcement menu items.


 0 kommentar(er)
0 kommentar(er)
